Can Syringe Filters Be Reused? What You Should Know
Syringe filters are essential in many scientific and industrial operations, influencing processes that range from critical lab experiments to large-scale production techniques. These devices keep liquids clean, free from particulates, and uncontaminated, facilitating accuracy and reliability in various applications.
As industries strive for cost-effectiveness and sustainability, reusing syringe filters presents a compelling opportunity.
However, there are multiple factors to consider, such as the material of the filter, potential contamination risks, and the specific requirements of each application. In this guide, you’ll find everything you should know about reusing syringe filters, clarifying whether this option is viable without sacrificing the quality and precision of operations.
Understanding Syringe Filters
Syringe filters are disc-like apparatuses attached to the end of a syringe to facilitate the removal of particles from liquids. Typically composed of a housing and membrane filter, the filters come in various pore sizes and membrane types to suit different needs.
Their primary function is to purify solutions by retaining particles larger than the membrane’s pores so that only the filtered solution passes through. This trait makes syringe filters indispensable in pharmaceutical labs, biological research, and chemical processes where purity is paramount.
The Importance of Materials and Construction in Reuse Viability
The materials and construction of syringe filters determine their performance, longevity, and reuse capability. These filters generally consist of a housing made from resilient polymers such as polypropylene, polycarbonate, or polyethylene, each selected for its unique chemical resistance and structural integrity.
The choice of housing material is crucial, as it affects durability and how the filter will interact with different chemical solutions. The precision in manufacturing and quality control employed during syringe filter production directly correlates with the reliability and consistency of filtration results. Understanding these details allows reused filters to maintain their integrity without compromising the purity and accuracy required in critical processes.
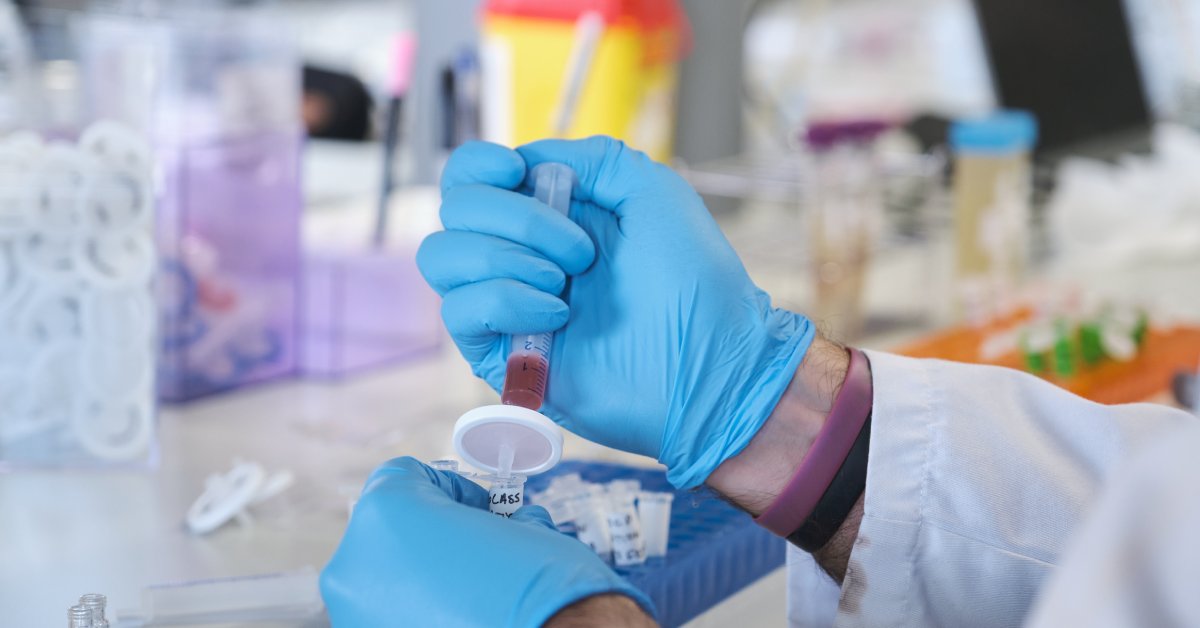
The Risks Involved in Reusing Syringe Filters
Reusing syringe filters can introduce several critical risks that could compromise your processes:
- Cross contamination: The primary concern with reusing filters is cross contamination. When reused, filters may retain particulate matter from prior filtration, potentially contaminating new samples. This residual carryover can alter results, damaging the reliability of experiments or processes.
- Membrane clogging: With each use, the chances of membrane clogging increase. Once clogged, the membrane cannot perform its filtration function efficiently, potentially allowing impurities to pass through into the sample.
- Reduction in structural integrity: Exposure to various chemicals and consistent pressure during reuse can weaken the syringe filter’s structural integrity.
- Membrane damage: The membrane’s ability to filter can deteriorate with each use, as continuous pressure and exposure to various chemicals can alter its porous structure.
- Chemical compatibility issues: Different filters handle specific chemical compositions. Reuse might introduce filters to incompatible substances, resulting in potential damage or ineffective filtration.
- Increased error potential: As filter performance degrades with reuse, the likelihood of errors increases. This degradation can compromise the validity of your operations, leading to a loss of time and resources to rectify inaccuracies.
This decline in efficiency emphasizes the need for single-use adherence to maintain the integrity of experimental and process outcomes. Incorporating quality plastic syringes into laboratory practices aids in maintaining purity, but the reuse of filters creates vulnerabilities like contamination and mechanical degradation.
Cleaning Syringe Filters
Cleaning syringe filters for potential reuse is intricate, demanding precision and thoroughness to remove lodged particles, residues, or contaminants effectively. The process requires flushing the filter using solvents or buffer solutions that are chemically compatible with the filter material. The choice of cleaning agent is crucial, as it must be capable of dissolving buildups without damaging the delicate filter membrane.
Depending on the material, ultrasonic cleaning or backflushing might be necessary for more stubborn contamination. Once the visible residues are gone, the filter needs rinsing multiple times to ensure no agents remain, which could otherwise lead to contamination during subsequent uses. Drying the filter completely prevents bacteria or mold growth.
Factors That Influence Syringe Filter Reusability
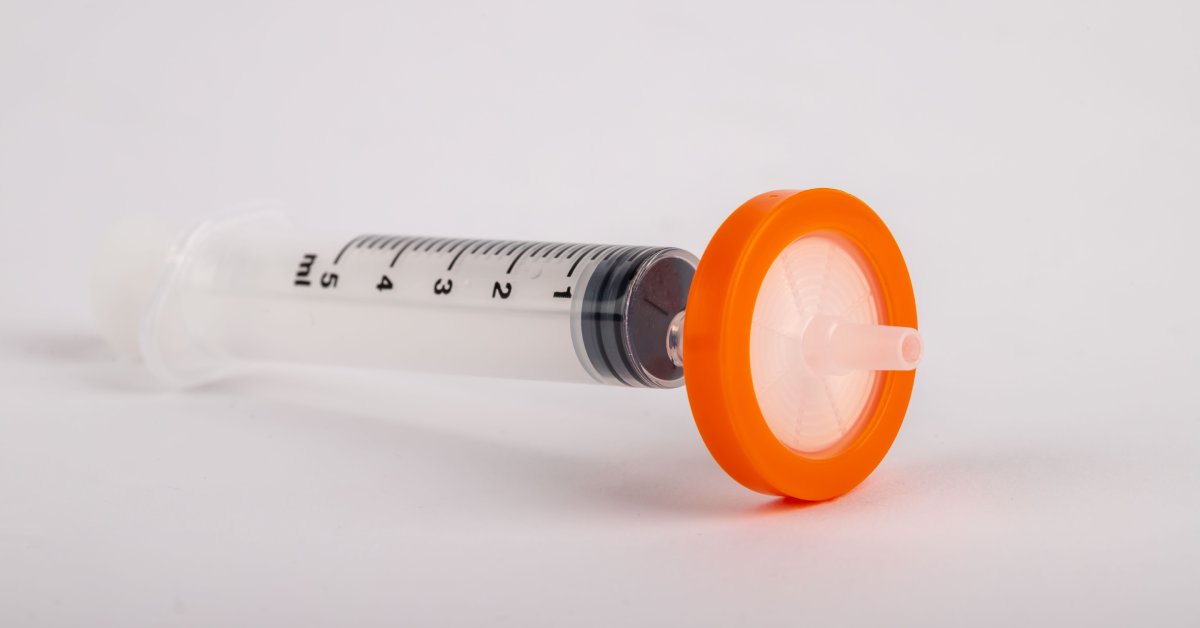
While reusing syringe filters is generally not the best option, certain conditions may allow for limited reuse. These include the type of liquid filtered, the volume processed, the particulate matter’s properties, and the filter material’s robustness.
Filtering nonviscous fluids with no chemical reactions may present situations where limited reuse is possible. However, assessing the risks on a case-by-case basis remains vital so reuse practices don’t compromise the outcomes’ integrity.
Environmental Considerations of Single-Use Syringe Filters
The environmental impact of routinely discarding single-use syringe filters is a pressing issue in laboratory and industrial settings, especially pertaining to sustainability and waste reduction. The reliance on syringes and filters designed for optimal performance results in significant plastic waste accumulation, making it imperative to explore eco-friendly alternatives.
Balancing environmental responsibility with the need to maintain high standards of purity and precision in filtration processes presents a challenging but necessary endeavor. Companies must prioritize environmental strategies with technological advancements and programs to make syringe filtration more sustainable while retaining the high performance required in critical applications.
Cost Implications of Syringe Filter Reuse
While reusing syringe filters might seem like a cost-effective strategy initially, it can introduce substantial hidden costs over time. The financial implications of compromised experimental outcomes due to contamination or reduced filtration efficacy might quickly offset the apparent savings from reduced filter purchases.
The labor and chemicals required for thoroughly cleaning and testing reused filters further add to operational expenses. Organizations must consider these factors and balance them against the presumed savings, recognizing that investing in high-quality, reliable single-use filters could prevent costly errors and operational inefficiencies.
Regulatory and Ethical Aspects To Consider
In various industries, adherence to stringent regulatory guidelines is necessary, especially concerning syringe filter usage, which directly impacts quality and safety standards. For sectors like pharmaceuticals, biotechnology, and food production, the integrity of these filters is nonnegotiable, as they safeguard against contamination and uphold product purity.
Neglecting protocols, such as reusing filters contrary to manufacturer guidelines, may not only breach legal regulations but also pose significant ethical risks, jeopardizing consumer safety and undermining trust. Compliance with industry standards and rigorous quality controls is essential to upholding ethical practices and safeguarding public health.
While there may be select scenarios where syringe filter reuse is viable, general recommendations emphasize the value of single-use filters for greater safety, efficacy, and reliability. Implementing best practices involves understanding the specific needs for these filters, investing in quality plastic syringes, and fostering a culture that prioritizes performance and safety.
Recent Posts
-
The Role of Desiccants in Protecting Hygroscopic Chemicals
Hygroscopic chemicals readily absorb moisture from the surrounding environment, leading to compromis …May 19th 2025 -
All About Pairing Containers With Corrosive Substances
Handling corrosive substances is critical in many industries, including manufacturing, pharmaceutica …May 12th 2025 -
Why Solvent Purity Is Crucial in the World of Chemistry
When producing accurate and reliable results in chemistry, solvent purity is non-negotiable. Many se …May 11th 2025




